PRESENTED IN EUROTOX 2023
Human kidney patient-derived organoids are a promising tool for modeling drug-induced nephrotoxicity and for assessing preclinical toxicity of immunotherapy
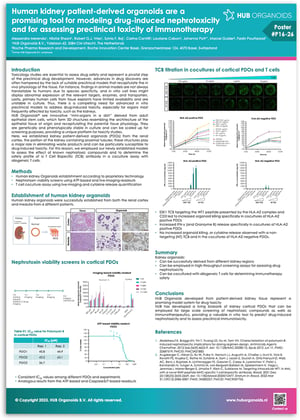
Preclinical toxicology assessments represent a pivotal step in preclinical drug development. However, advances in drug development are often hampered by the lack of suitable preclinical models that recapitulate the in vivo physiology of the tissue. For example, animal models are not always translatable due to specified specificity, in vitro cell lines might display abnormal expression of targets, enzymes, and transport proteins, and primary human cells from tissue explants have limited reproducibility due to availability and are unstable in culture. HUB Organoids are derived from adult epithelial stem cells and recapitulate the 3D architecture of the epithelial tissue of origin. They are genetically and phenotypically stable in culture and can be scaled for screening purposes, providing a unique platform for toxicity studies.
Download this poster to discover:
- The establishment of patient-derived organoids (PDOs) from the renal cortex, a key kidney structure responsible for eliminating waste products and prone to drug-induced toxicity.
- The development of a high-throughput screening assay for assessing drug nephrotoxicity.
- The creation of a PDO-based allogeneic T cell co-culture assay for evaluating immunotherapy safety.

